Safety first because injuries last

As a prelude to our forthcoming IEC 61010 training courses, we take a look at the similarities and differences between the IEC 61010 and the IEC 60601 series of standards, and going further the reasons why in certain areas, particularly electromagnetic compatibility (EMC) the latter is preferred.
The IEC 61010 series of safety standards is applicable for the safety requirements for electrical equipment for measurement, control and laboratory use. In theory independent from the requirements for Medical Electrical (ME) Equipment governed by the IEC 60601, where the main focus of the IEC 60601 series is preventing harm to patients, either connected to the ME Equipment through an applied part, or where energy is transferred to the patient or measured from the patient. The focus of the IEC 61010 series is on electrical tests and measurement equipment, electrical process-control equipment and electrical laboratory equipment.
What sets the IEC 60601 series apart from the IEC 61010 series is the potential higher risk of electrocution through low impedance contact to a patient. IEC 60601 places a greater emphasis on risk analysis and includes a section on Programmable Electrical Medical Systems (PEMS), which is the first step towards assessing embedded systems and the functional safety thereof.
Although omitting certain clauses listed above, there are aspects of the IEC 61010 series that address key safety-related items in a more concise and informative manner. As covered in our blogs „Isolation distances are not so creepy!“ and „Cleaning Up Your MOPPs“, the electrical safety challenges in IEC 60601 manifest themselves in two categories – Means of Operator Protection (MOOP) and Means of Patient Protection (MOPP). Due to the lack of an applied part in contact with a patient, IEC 61010 focuses on the former. Over the years of running our IEC 60601 training courses, one topic that crops up time and time again is electrical safety in section 8. Many people find the 65 page section and subsequent annexes confusing, which is not surprising as there is a huge amount of information packed into the section.

Take the first step towards mastering IEC 61010 by contacting our experienced consultants: info@lorit-consultancy.com. Let us take your skills to the next level!
Erfahre mehrFigure 1 below indicates the graphical representation of means of protecting against electrical shock from IEC 61010. This representation would be helpful in IEC 60601, as it represents how individual means of protection can be built up to represent the required two means of protection.
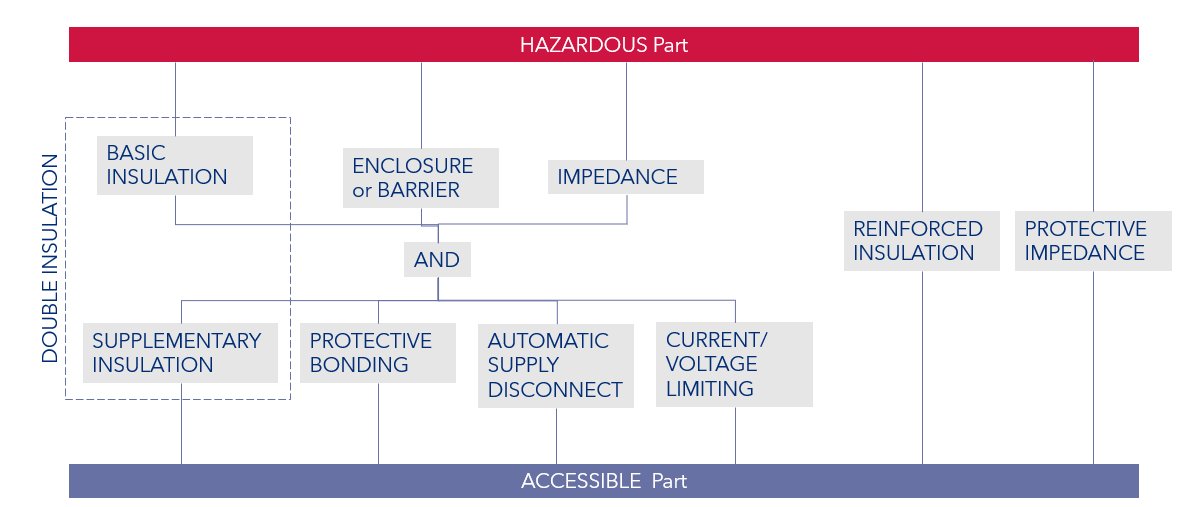
Figure 1: Protective means against electric shock
The categories of mechanical hazards in the two series are fairly similar, but there some areas that are better handled in IEC 61010 e.g., insultation with in printed circuit boards and the requirements associated with preventing the spread of fire as indicated in Figure 2.
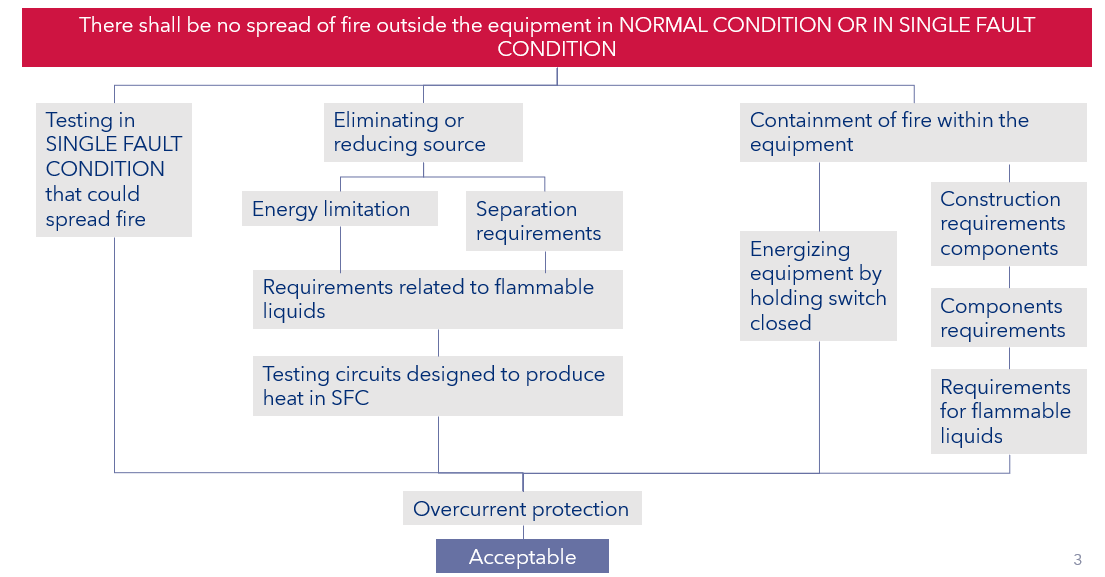
Figure 2: Protective against the spread of fire
One key difference between the two series of standards are the requirements for EMC testing. IEC 61010 utilizes the IEC 61326 series of EMC standards, which have less stringent test limits than those defined in IEC 60601-1-2, the corresponding EMC standard for ME Equipment. This is one of the reasons why the FDA recommends testing IEC 61010 relevant devices to the limits defined in IEC 60601-1-2 in Electromagnetic Compatibility (EMC) of Medical Devices Guidance for Industry and Food and Drug Administration Staff June 6, 2022. The guidance goes further by recommending maximum test levels based on the intended EMC environment. A tip that applies to any EMC assessment as the environment may produce greater threat levels than would be tested by following the limits in the standards. The pain of electrostatic discharges impacting devices at trade shows, based on the choice of flooring and low humidity is something many have experienced!
The EMC challenge goes further with the FDA also looking for compatibility with RFID readers, guidance on this is provided in the FDA consensus standard AIM 7351731 Medical Electrical Equipment and System Electromagnetic Immunity Test for Exposure to Radio Frequency Identification Readers. Updates to IEC 60601-1-2 in 2020 brought these two documents closer together in their guidance, but there are still some differences around electric fields.
By Alastair Walker, Consultant & Owner
To learn more about IEC 60601 or enroll in our new IEC 61010 training course, feel free to reach out to us at info@lorit-consultancy.com. Don’t hesitate to get in touch with us for further information.