When in vitro met functional safety

A subject often covered by us, is the concept of using state of the art in reducing risk in projects. A relevant subject that features in many industries is functional safety, i.e. the consideration of the safety of active devices Why a functional safety standard is needed in the medical device sector. Our focus is often on the Programmable Electrical Medical Systems (PEMS) section of IEC 60601 Making Sense of PEMS, however in the world of in vitro devices recent developments in IEC 61010-2-101 (the particular standard for in vitro medical equipment) have brought a focus on functional safety.
Annex J of IEC 61010-2-101 shines light on using different techniques for reducing risk and better still a combination of techniques to evaluate risk. The two functional safety standards in focus being EN IEC 62061 and EN ISO 13849. This then brings the concepts of Safety Integrity Levels (SIL) and Performance Levels (PL) into consideration for developing acceptable (in terms of risk) in vitro devices.
The good thing in annex J is the encouragement of considering a combination of techniques and that functional safety method will not address all concerns. There are many passive safety and human factors considerations too! The examples listed, where functional safety could play a role are as follows:

If you would like to find out more about functional safety in relation to in vitro devices and IEC 61010 using EN ISO 13849 or EN IEC 62061 please join one of our IEC 61010 training courses, likewise we cover the subject in detail in our IEC 60601 Advanced course. Send us your inquiry to info@lorit-consultancy.com.
Learn morea) monitoring the position of mechanical safety devices;
b) monitoring of the monitoring devices;
c) plausibility testing (e.g. monitoring the function of redundant electronic systems);
d) self-testing of measurement systems.
The flow chart of figure 1 indicating the different approaches based on the IEC 61010 standards series and the functional safety approach.
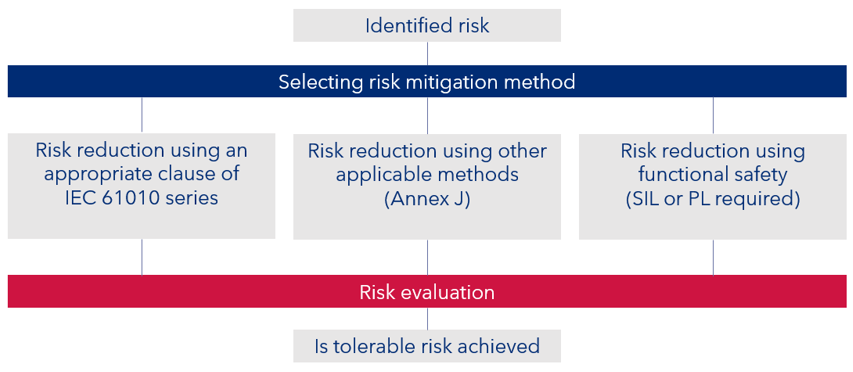
Figure 1 – Risk reduction using a combination of techniques
The use of EN IEC 62061 and EN ISO 13849 opens a major can of worms for teams more familiar with the design of in vitro devices. The two standards address the functional safety of machinery and more specially control systems.
SILs being defined based on a top-level hazard identification, this then defines the subsequent rigor at system, hardware and software levels. PLs define the ability of safety-related parts of control systems to perform a safety function under foreseeable conditions are allocated one of five performance levels.
Figure 2 indicating the SILs and PLs based on the level of risk
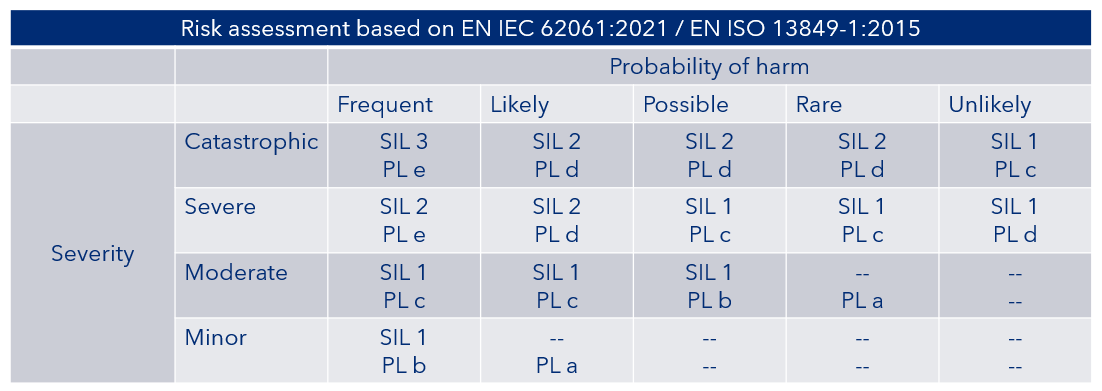
Figure 2 SIL and PL requirements based on risk
As with all functional safety standards, both bring a variety of key considerations in scope:
There is no real meat on the bones in annex J of IEC 61010-2-101, but this is also the case in the PEMS section of IEC 60601, but as functional safety is such a large subject area it would not be practical to cover the topic in detail in an in vitro device standard. Annex J is also informative so introducing the state of art topic and encouraging the reader to delve deeper.
If you are interested in the subject of functional safety, then a detailed understanding of EN IEC 62061 or EN ISO 13849 would be required, but many embedded engineers who attend our training courses are already au fait with the subject, and this is important particularly in identify requirements for programmable devices such as microcontrollers. Most safety relevant microcontrollers on the market these days are accompanied by a safety manual that helps shed light on the quality of e.g. diagnostic coverage or built in self-test. This helps in the decision-making process when selecting the device.
By Alastair Walker, Consultant & Owner
If you would like to join one of our IEC 60601 Advanced or IEC 61010 training courses, please do not hesitate to contact us at info@lorit-consultancy.com